A Legal Opinion of What You Can and Cannot Say
This is a reoccurring topic of discussion at The CBD Expo Tour, therefore this is a fluid post that we are continually updating. We have participated in multiple shows on the same panel discussion and want to ensure we provide the newest and most diverse points that are made.
The panel in Denver, May 7-8th was with Kristen Nicholas as the moderator and panelists including Michael Minardi, Esq, Jessica Arent from Cannabidiol Life, Liz Geisleman with 710Spirits® and Fenella Keig a CBD compliance and GMP consultant.

On the panel in Indianapolis, April 8-9th was with moderator Jessica Arent, panelists were Liz Geisleman from 710Spirits®, Ronie Schmelz Class Action Defense Lawyer at Tucker Ellis LLP, and Blair Curless Pharm. D, Ph.D. clinical pharmacist at Phoebe Putney Health System.

Basics
As legalization takes hold nationwide it is incredibly important to make sure you and your company understand the guidelines of labeling and claims for your product. Understanding the rules and standard procedures to follow will help create a solid foundation moving forward. Ensuring longevity for your company and product. The FDA and FTC are the institutions to always be aware of. Make sure to understand what they are looking for in claims and marketing real estate.
FDA
The Food and Drug Administration (FDA) is focused solely on certifications and ensuring that consumer products being put out to the public are safe, while accurately stating what the product will do. If you are making false claims the FDA will typically send out a warning letter that outlines what your companies infraction is on. The manufacturing of product, customer complaints, disease/medical claims that could designate your product as a drug, for example.
A company then has 15 days to respond following the letter of specifically what and how to correct such claims. Some have also received Covid-19 warning letters that have only allowed 48 hours to respond, so watch those claims!
FTC
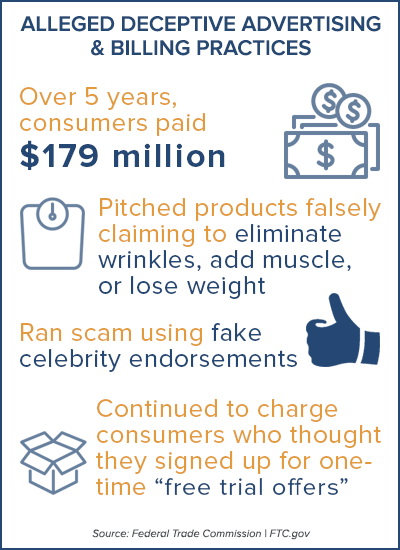
While the Federal Trade Commission (FTC) focuses on extravagant medical claims. Statements made by your product in all aspects of your marketing real estate and can administer a fine immediately. These two legal entities watch every claim being made especially by companies in the CBD industry.
Essentially the overarching lesson from all the panelists was that you cannot say anything that your product remedies without being able to substantiate it. (Unless you have applied for a new drug through the FDA and it has been approved!) Making claims that “cure” ailments will get you or your company into hot water. Allegations such as anti-aging, skin clearing, inflammation, chronic pain, soreness etc. are not going to fly. Everything should be stated as a treatment but not as a cure. Think health and wellness not disease and sickness.
Marketing
The larger the company, the more money they have backing a claim, ergo the more likely the risk tolerance. Some companies have risk tolerance even added into their marketing budget. Under the circumstances it is up to you and your business what you are willing to risk.
In this day and age it is not difficult to appear as a well-established business with vetted practices and quality sourced products. Especially with technology and freelance website builders; it can cost a grand to appear like a million dollar organization. Always know who has access to your site! Make sure that you trust the people writing the copy as it represents your company. Consequently it is our responsibility as those creating the foundation for this industry to self-enforce. Ensuring we are marketing and providing safe products to all of our customers.
What To Watch Out For
Always always always remember that you are responsible for your marketing real estate! Meaning anything put on your labels, website, advertisements, or social media will be scrutinized by the FTC and FDA.
Social Media
If you are using social media influencers make sure to get a solid contract signed. And always verify all posts before having them go live. Even though it is not an employee making statements about your product they are representing your brand. Their statements are your company’s statements (INCLUDING HASHTAGS). Don’t interact with social media posts or comments that make medical claims. While the exposure could be great for your company even a like or response on a separate social media account connects the product to the claim and therefore your company.
Reviews
This extends to customer reviews as well. Reviews have become gold in the digital age but make sure none of the reviews you are putting on your site are making claims you cannot back up. For example, if a review states “Amazing product! My acne is gone, and I no longer deal with sleep issues.” Putting this review on your site, even stated by a third party, is your company backing the claim that your product cures and therefore is a drug.
Research
Moreover be aware of using research connecting to your product. For instance if your product contains Vitamin C and you then link a research paper illustrating the positive effects of Vitamin C this can make you liable as well. Linking research to a component of your product means making sure that the numbers match up. Conditions such as:
- having the same demographics in the study as your target audience
- same dosage in the study as in your product
- other ingredients that might counteract the claim in the study
- bonus points if you were a part of the study
Temp Terms
Using temporary terms is something to keep clear of as well. Suggestive language such as “could, might, etc.” are a risky game and while you might be able to get away with it the possibility of getting fined is large. Words such as “natural” and “organic” also have not officially been defined by the FDA and FTC. This means you can use the term but again it’s dangerous. Eventually the terms will be defined and if you aren’t in the area of the definition falls under you could be fined later down the line.
The FDA has guidelines here on what terms they have ok’d (link is for dietary supplements but they also have for food and drug terms). Still, the FDA has fine tuned the process of scouring hundreds of websites in a day for claims so always make sure to keep up with the warning letters on the FDA website! Maintaining a solid foundation moving forward that adhere to their guidelines can create a trusted brand and longevity in the company.

Legal Steps
It is always about the spectrum of your company’s risk tolerance but if your risk tolerance is high and you do get hit with a FDA warning letter here is what to expect. You will receive a consumer demand letter with a 15 day notice. Then you will be sued for monetary relief. The FDA might also send a letter for drug claims. Your company will be put on the FDA site and you are at risk for being cut off from banking and black listed as well.
They most likely will calculate out how much retail value in the last x amount of years you have procured, and you can be sued for that amount. Keep in mind they can also sue for multiple states or nationwide. Especially, if you have sold through ecommerce.
It is your job to always review annually what your local and state laws are. It is your burden to know!
The FTC has a list on their site of what fines they are giving out on what claims. While the FDA also has a list of warning letters issued. Both are a great resource to reference for what organizations and consumer lawyers are focused on.
The following article goes through a warning letter itself and addresses many claims made. It is a great source of information and a good read as well. Compliance Lessons: In-Depth Warning Letter Discussion
We’re still figuring it out
There is still a huge gray area with the industry being so new. The consensus is that unfortunately it is going to get worse before it gets better. Because it is so new there is no established overarching guideline to follow. The rules and regulations will continue to change and update as the industry grows.
Always be willing to adapt and pivot to continue your business forward in the most ethical way with the best practices you can. Establish yourself as a strong business and brand moving forward.
To answer questions and concerns, always make sure to get a lawyer or consultant’s opinion. If you are looking for a consultant, check out founding member Allay Cannabis Consulting for any questions you might have!
Works Cited
Center for Food Safety and Applied Nutrition. “Structure/Function Claims.” U.S. Food and Drug Administration, FDA, www.fda.gov/food/food-labeling-nutrition/structurefunction-claims.
Commissioner, Office of the. “Warning Letters and Test Results for Cannabidiol-Related Products.” U.S. Food and Drug Administration, FDA, www.fda.gov/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products.
Commissioner, Office of the. “FDA Regulation of Cannabis and Cannabis-Derived Products: Q&A.” U.S. Food and Drug Administration, FDA, www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd.
Federal Trade Commission, 14 Apr. 2021, www.ftc.gov/.

Teagan is the Marketing & Communications Manager for 710Spirits® and Rocky Mountain Reagents. While continually learning about the cannabis industry and all the possibilities it has to offer she creates content for The Canna Consortium both for blog posts and social media. Make sure to check out @thecannaconsortium2.0 for all industry updates and news.